Fighting the Syphilis and HIV Epidemics: Voices from the Field – NCSD Webinar
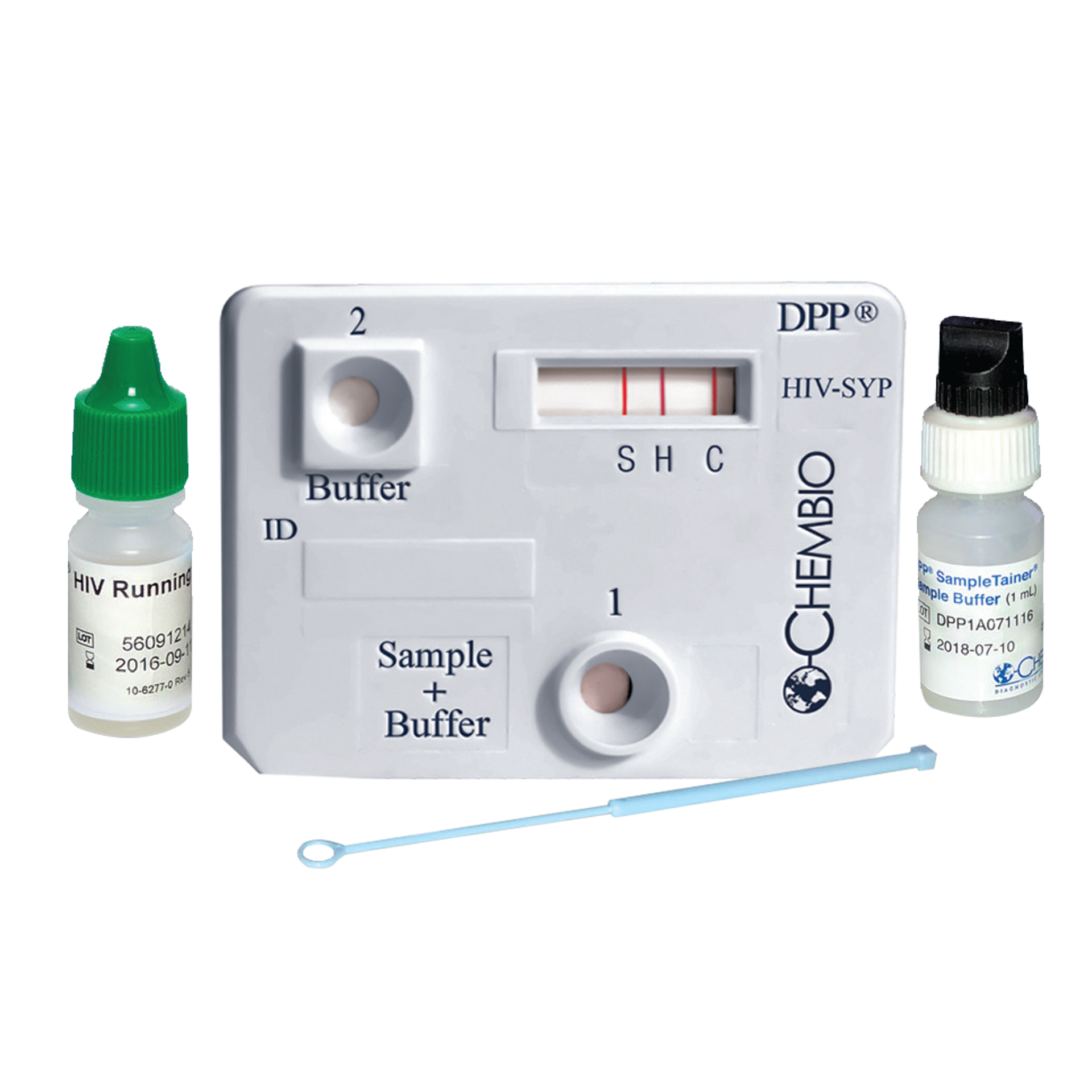
DPP® HIV-Syphilis Assay

CE-Marked

FDA Approved
- Patented DPP® technology allows for higher sensitivity and specificity
- 2 test results in a single sample
- Small sample volume: 10 μl
- DPP sample tainer included for safe and closed sample handling
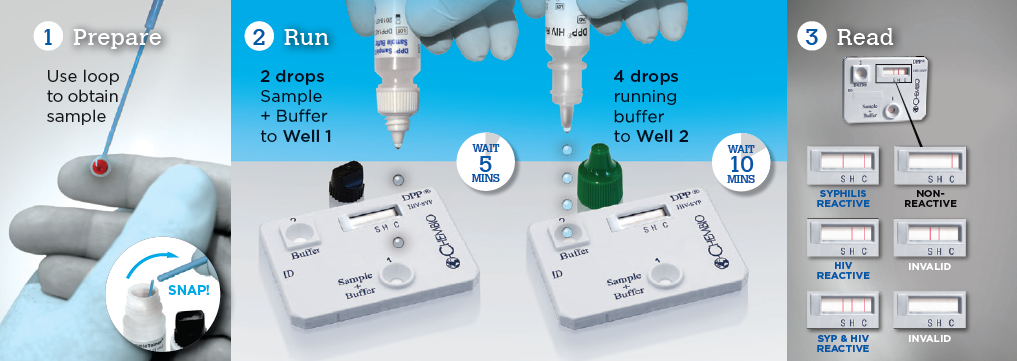
A dual rapid test for the detection of antibodies to HIV 1/2 and Treponema pallidum in fingerstick whole blood, venous whole blood, serum or plasma specimens.
| Platform | Immunochromatographic Assay |
| Format | Cassette |
| Detection | HIV-1 and HIV-2 antibodies |
| Specimen | Whole blood, serum & plasma |
| Assay Time | 15 minutes |
| Shelf Life* | 24 months |
*From date of manufacture
Dual Path Platform
Chembio’s proprietary DPP® technology differs from classical lateral flow tests by operating in a manner similar to that of the sequential ELISA format which is not sensitive to the “Hook Effect”.
DPP® Technological Advantages:
- Enhanced multiplex capability up to 8 biomarkers with results in minutes
- Significantly increased analytical and clinical sensitivity
- Ability to effectively resolve normal aggregation/agglutination migratory issues, a common concern in lateral flow assays with large particle analytes (e.g., bacteria)
- Adaptable to multiple sample types such as fingerstick blood, oral fluid, venous whole blood, serum and plasma
Do you still have questions?
For Technical Support please call
1300 418 188 9am-7pm AEST / 9am-8pm AEDT, 7 days per week
Or contact us via the form below and a member of the team will get back to you.
To contact your local state/territory health department click on the following link:
https://www.health.gov.au/about-us/contact-us/local-state-and-territory-health-departments